
The Complexities of “Simplification” – Using a Single IRB to Review for Multiple Sites
October 2017 Issue
Authors:
-
Fanny K. Ennever, PhD, CIP
Manager, Regulatory Policy Development
Human Subject Protection Program
Boston Medical Center and Boston University Medical Campus
- Introduction
- Advantages of Single IRB Review
- Challenges of Single IRB Review
- Implications for Lead Study Team
- Implications for Local Study Teams
- Summary
Introduction
The Federal regulations have always allowed a single IRB to review a protocol that takes place at more than one site, but there is an increasing push to make single IRB review required. All NIH-funded multi-site studies will be required to use single IRB review if they have a receipt date for competing grant applications (new, renewal, revision, or resubmission) on or after January 25, 2018. Other federally-funded multi-site studies will be required to use single IRB review after January 19. 2020.
Single IRB review makes some processes more efficient, but also introduces several complexities because the institution where the research takes place (the “Relying Institution”) retains or shares several responsibilities with the single IRB, as described in this article.
Advantages of Single IRB Review
Single IRB review has three main advantages:
- The protocol is only reviewed once. This avoids delay and inconsistencies compared to having IRBs from each investigator’s institution review the protocol.
- The single IRB may have greater leverage with sponsors to require changes. With multiple-IRB review, if any individual IRB asked for a change, the sponsor could just decline to run the study at that institution. A sponsor could not just ignore the stipulations of the single IRB.
- The Lead Study Team (the study team with the overall responsibility for the protocol) submits amendments and continuing reviews to the single IRB. This reduces the paperwork for the local study teams.
Challenges of Single IRB Review
Coordination between the single IRB and the relying institution: In order for the single IRB to carry out its review, it must coordinate responsibilities for:
- Local context review. The single IRB must be able to take into account specific requirements of state and local laws.
- Local requirements. The single IRB must establish a mechanism for ensuring that the investigator has:
- Obtained required local signoffs by Nursing, Radiation Safety, etc.
- Completed local training and Conflict of Interest disclosure
- Complied with local recruitment policies for non-English speakers, limited- and non-readers, etc.
- Incorporated locally-reviewed language into the consent form for compensation for injury and costs
- Post-approval monitoring. The single IRB must coordinate post-approval monitoring with the relying institution, including both for-cause and not-for-cause audits.
Increased responsibilities of the Lead Study Team: The Lead Study Team becomes the liaison between the single IRB and the local study teams at all other sites, and must communicate:
- Requirements for:
- submitting to the single IRB for initial site approval
- providing continuing review information
- reporting unanticipated problems, deviations, etc. to the single IRB
- All determinations of the single IRB
- The overall protocol and site-specific consent forms and recruitment material, including any changes resulting from amendments
New standards to learn for Local Study Teams: When the Local Study Team is carrying out a study that is reviewed by a different IRB, they are responsible for learning:
- The process for requesting single IRB review from the BMC/BU Medical Campus IRB
- The process for requesting to join the study from the single IRB
- The reporting requirements of the single IRB for unanticipated problems, deviations, etc., which are almost certainly different from the BMC/BU Medical Campus IRB reporting requirements
Implications for Lead Study Team
As soon as Principal Investigators are aware that they are going to be the Lead Principal Investigator on a multi-site study that is required to use single IRB review, they must contact the BMC/BU Medical Campus IRB. Except for small, simple multi-site studies, we do not have the resources to act as the single IRB, so we will help identify an appropriate single IRB. The budget must include funds for the single IRB as well as adequate staff to carry out the responsibilities of the Lead Study Team.
The Lead Study Team is responsible for managing the process for identifying sites and initiating the process for establishing the reliance agreement between each relying institution and the single IRB. There is a nationwide reliance agreement electronic system called “SMART IRB” to facilitate this process which the Lead Study Team will have to learn how to use.
Implications for Local Study Teams
Initial submissions: The process for requesting to join a study with single IRB review starts with submitting the request (called a “cede review” request) through INSPIR. There is a separate review path that is chosen in application section 4:
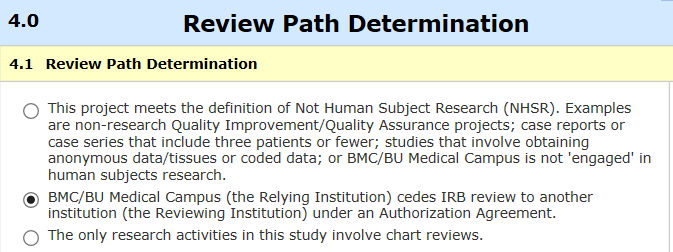
Information required on this path includes the protocol, identifying the single IRB, use of drugs or devices, recruitment of special populations (students/employees, cognitively impaired, non-English speakers, and limited- or non-readers), and consent form “Compensation for Injury” language. When the cede request is accepted, INSPIR will send a formal letter to the study team.
Continuing obligations to BMC/BU Medical Campus IRB: Most submissions after initial approval are made to the single IRB. The exceptions are:
- Study personnel changes, which first must be submitted through INSPIR (so BMC/BU Medical Campus IRB can check for required training and Conflict of Interest disclosures). The INSPIR approval letter is then submitted to the single IRB to change study personnel.
- Internal Unanticipated Problems, which must be submitted simultaneously to the single IRB and through INSPIR (so BMC/BU Medical Campus can start any needed action without waiting for the report to be processed by the single IRB).
Summary
Being part of a multi-site study comes with additional responsibilities. The Local Study Team needs to know how to follow the policies of the reviewing IRB and the BMC/BU Medical Campus IRB. Single IRB review has significant advantages but does not make life simple, just different.