Recruiting for an Outside Study: When and How to Submit to the IRB
September 2018 Issue
Authors:
- Fanny K. Ennever, Research Compliance Officer, BMC
- Matthew T. Ogrodnik, IRB Director
- Introduction
- How do I know whether I’m “involved” (“engaged”) in the study?
- When is IRB submission required even when I am not “involved” (“engaged”)?
- What is meant by release of PHI outside the covered entity?
- How do I submit?
- How do I fill out the HIPAA section?
- What are some examples?
- What if I’m not sure?
Introduction
Sometimes you may be approached by a colleague at another institution who wants to recruit BMC or BU Goldman School of Dental Medicine (GSDM) patients for their study. If you or another investigator from BMC or BU Medical Campus is also involved in the study, then the normal process for obtaining IRB approval applies. This article addresses the situation where no one from BMC or BU Medical Campus is involved in the study.
How do I know whether I’m “involved” (“engaged”) in the study?
If you will have contact with subjects or their identifiable information after they’re recruited, you are involved in the research. If not, the INSPIR system has a pathway for submission even when you are not involved – it’s called “not-engaged,” and is a determination that is made when research that does meet the definition of human subjects research is going on at some institution, but when none of the activities at BMC or BU Medical Campus meet that definition (see Engagement in Research).
The BMC/BU Medical Campus HRPP policies follow federal guidance in specifically listing four recruitment activities that do NOT constitute engagement in research:
- Informing prospective subjects about the availability of the research
- Providing prospective subjects with information about the research (which may include a copy of the relevant informed consent document and other IRB-approved materials) but not obtaining subjects’ consent for the research or acting as representatives of the investigators
- Providing prospective subjects with information about contacting investigators for information or enrollment
- Seeking or obtaining the prospective subjects’ permission for investigators to contact them
When is IRB submission required even when I am not “involved” (“engaged”)?
There are three situations where submission to the IRB would be required for recruitment for outside studies:
- If the recruitment specifically targets students, trainees, or employees of BMC or BU Medical Campus
- If the recruitment involves direct contact with BMC or BU GSDM patients by an outside researcher
- If the recruitment involves the release of Protected Health Information (PHI) outside the covered entity (BMC or BU GSDM)
Submission to the IRB in categories 1 and 2 will allow the institution to evaluate whether the research is appropriate for our students, trainees, or employees (#1) or our patients (#2).
Submission to the IRB in category 3 is required because of the institutional responsibility to oversee the release of PHI under HIPAA. The criteria for whether recruitment activities fall into category 3 are somewhat complex.
What is meant by release of PHI outside the covered entity?
The BMC and BU Medical Campus HRPP considers that PHI has been disclosed outside of the covered entity when PHI from BMC or BU Medical Campus is used to determine that a patient is eligible for a study, and this fact is directly communicated to a researcher outside BMC or BU Medical Campus. The key is “directly communicated.” If the recruitment activities only involve the patient initiating contact with the outside investigator (e.g., calling a number on a brochure), then the covered entity (BMC or BU GSDM) is not releasing the information. In contrast, direct communication of PHI would be involved if someone here is in possession of health information that indicates that the patient may be eligible. In this case, when that person communicates the patient’s permission and contact information directly to the outside investigator, then that person is also communicating the health information that the patient is eligible, thus releasing PHI outside of BMC or BU GSDM.
The determination that PHI is being released is highly dependent on the details of the recruitment activity. We don’t expect investigators to be experts -- just to know that they should call or email the IRB if they have any questions about what is required when assisting in the recruitment for an outside study.
How do I submit?
Finding the “not engaged” submission is a little tricky: it’s on the fourth INSPIR screen:

You are then taken to the fifth screen, where you answer “No” to the first question:

You will have to answer two more questions on this page describing the recruitment activities, and a few questions on the subsequent page about funding for the project. You are then taken to the HIPAA section.
How do I fill out the HIPAA section?
Short answer: contact the IRB for help. If you would like to try by yourself, then there are two main paths:
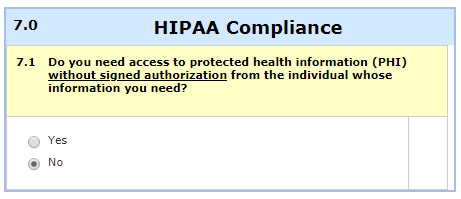
- You will obtain signed HIPAA authorization from the prospective subjects: In this case, you attach the authorization and the HIPAA section is simple:
- You will not obtain signed HIPAA authorization from prospective subjects (this could occur if the contact is over the phone or is in a setting where going over the entire authorization would be overly burdensome): You answer “Yes” to the above question, and then eleven other questions providing a justification for why it’s not possible to obtain an authorization.
What are some examples?
1) An outside investigator at MGH is conducting a clinical trial for patients with congestive heart failure. The MGH investigator has contacted his colleague at BMC and asked if she would be willing to distribute recruitment brochures to her patients in clinic who may be eligible and interested. The brochures contain contact information for the investigator at MGH, and patients can call if interested. The only role of the BMC physician is to inform her patients about the study and provide the brochure.
Answer (UPDATED 10/16/2020): It is possible that no submission to the IRB is needed. In this example, the activities fall under criteria #1-3 that were described earlier in the article, and there is no release of PHI outside the covered entity. The patients are calling the outside investigator directly using the contact information from the brochure. There may be circumstances, however, when the IRB should be involved in the review of this scenario (if, for example, the study involves a particularly sensitive topic). The best thing to do is check with the IRB by sending an email to medirb@bu.edu. Please also note that recruitment materials for outside studies distributed in BMC or BU Medical Campus space still need to be reviewed by the appropriate Communications Department. Please submit the materials according to the process described in the Research Study Recruitment Promotion – Toolkit.
2) An outside investigator at Tufts Medical Center is conducting a clinical trial for patients with chronic pain. She has asked her colleague at BMC to distribute study brochures to his patients and describe the study to them. If a patient expresses interest, then she is asking her colleague at BMC to share the patient’s contact information with her. She will then call the patient to explain the study and administer an eligibility screening checklist.
Answer: This scenario requires a submission to the IRB because protected health information is being released outside the covered entity. When a BMC physician provides the contact information for BMC patients directly to the outside investigator, he is also providing the information that this patient may be eligible for the study because of chronic pain (health information). Thus, the IRB needs to review the request to either obtain authorization from these patients or request a waiver of authorization.
What if I’m not sure?
Contact the IRB (617-358-5372 or medirb@bu.edu). We know this is a complex situation and are happy to help.